Product Overview
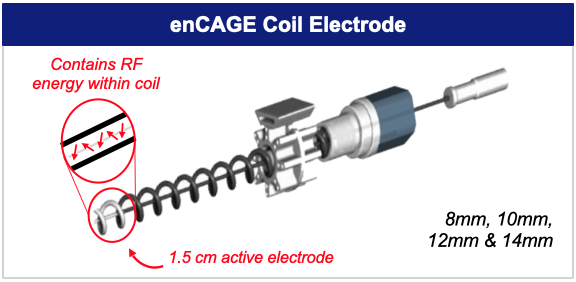
The enCAGE Coil is designed to eliminate the limitations of other methods of prostate cancer treatment that can cause erectile dysfunction and urinary incontinence. The device is a disposable, focal therapy, precision ablation system that will deliver bipolar, radio frequency energy through a distinctive coil electrode, or “cage”. It enables the surgeon to preset margins for precise tissue targeting. Similar to the principle of the “Faraday Effect”, the enCAGE coils retains the RF energy within the confines of the coil, preventing leakage beyond the ablation zone that could otherwise damage adjacent, vulnerable structures. The procedure is minimally invasive and can be performed in the hospital or a physician’s office.
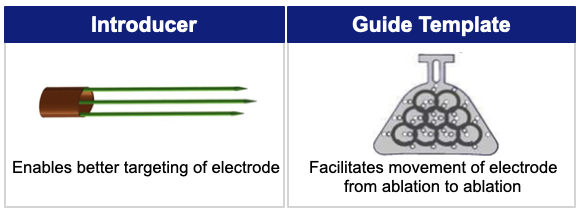
Electrodes, Introducer and Guide Template

enCAGE: The New Standard of Care for Prostate Cancer

Today’s Challenges
Important nerves immediately adjacent to tumors can be damaged.
Difficulty in controlling the treatment energy leads to energy damage beyond the margins of the tumor.
Erectile dysfunction and urinary incontinence are frequent side effects in these modalities.
The New Standard of Care: enCAGE Therapy
enCAGE Coil creates a Faraday Cage effect: “Walling off” the delicate structures.
Radio Frequency (RF) Energy, with the coil, targets the tumor.
Only the cancer is ablated Erectile & Urinary function are unimpaired.
Minimizes future costs to the healthcare system.

enCAGE Therapy Clinical Study
A clinical study was completed using the current device to ablate prostate tissue in 20 patients at University College London. Dr. Mark Emberton, Dr. Clement Orczyk. The study was published in The Journal of Urology which made the following editorial comment.
“Coil RFA with the enCAGE device is a unique option for focal prostate ablation. With transperineal insertion, and ablation limited to the coil cage, treatments can be applied to both anterior and posterior lesions with minimal chance of ablating nearby vital structures.
In this series of 20 men with intermediate risk prostate cancer, no grade 3-5 adverse events were reported, and 15 men had complete absence of any cancer at the 6-month biopsy. This is impressive considering that it was the first of its kind experience and post-treatment biopsy was mandated and thorough, with a median of 6 cores from the treatment site, which is beyond the approach of most focal therapy series. The success may in large part be due to the ingenious approach of adding extra needles to pull the energy outside the coil…”
Prostate Cancer Statistics
-
1 out of 7 men will be diagnosed
-
3.1M Living with Prostate Cancer
-
268K New Cases/year
-
34K deaths annually
-
$5-8B/Year costs to Medicare


