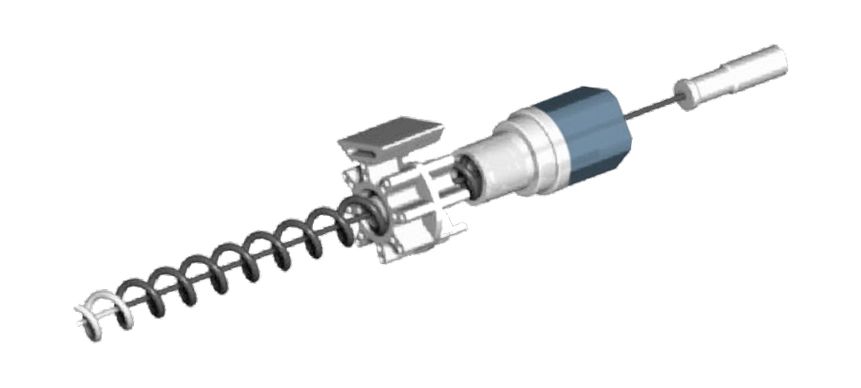
Imagin’s second technology, the enCAGE Coil, is being refined as a disposable, focal therapy precision ablation device that will deliver bipolar, radiofrequency energy through a distinctive coil electrode, or “cage”.
The system’s features will address the limitations of other forms of prostate cancer treatment by allowing the surgeon to pre-set precise margins to target only the cancer cells, potentially avoiding total removal of the prostate gland and damage to the adjacent erectile nerves that can cause impaired urinary and sexual function. The procedure will be minimally invasive and will be performed in a physician’s office. Imagin acquired Trod Medical and the enCAGE device in August 2022.
Imagin Medical, Inc. is a development stage company and the enCAGE Coil does not currently have any medical device regulatory approvals or clearances to market products for prostate cancer.
Prostate Cancer Statistics
Prostate Cancer is the most prevalent* cancer in men in the U.S. and the second leading cause of cancer deaths**
- 1 out of 7 men will be diagnosed
- 3.1M living with Prostate Cancer
- 248,530 new cases/year
- 314,130 deaths annually
- $1.2B prostate cancer cost to Medicare
CURRENT TECHNOLOGY
Today’s Treatment Challenge
Depending on the stage of the cancer and the age and overall health of the patient, doctors will recommend a variety of different treatments, most commonly surgery and radiation, followed by focal therapy.
Surgery is a common choice if the cancer has not spread outside the prostate gland. The main type of surgery for prostate cancer is a radical prostatectomy which involves removing the entire prostate gland plus some tissue and nerves around it.
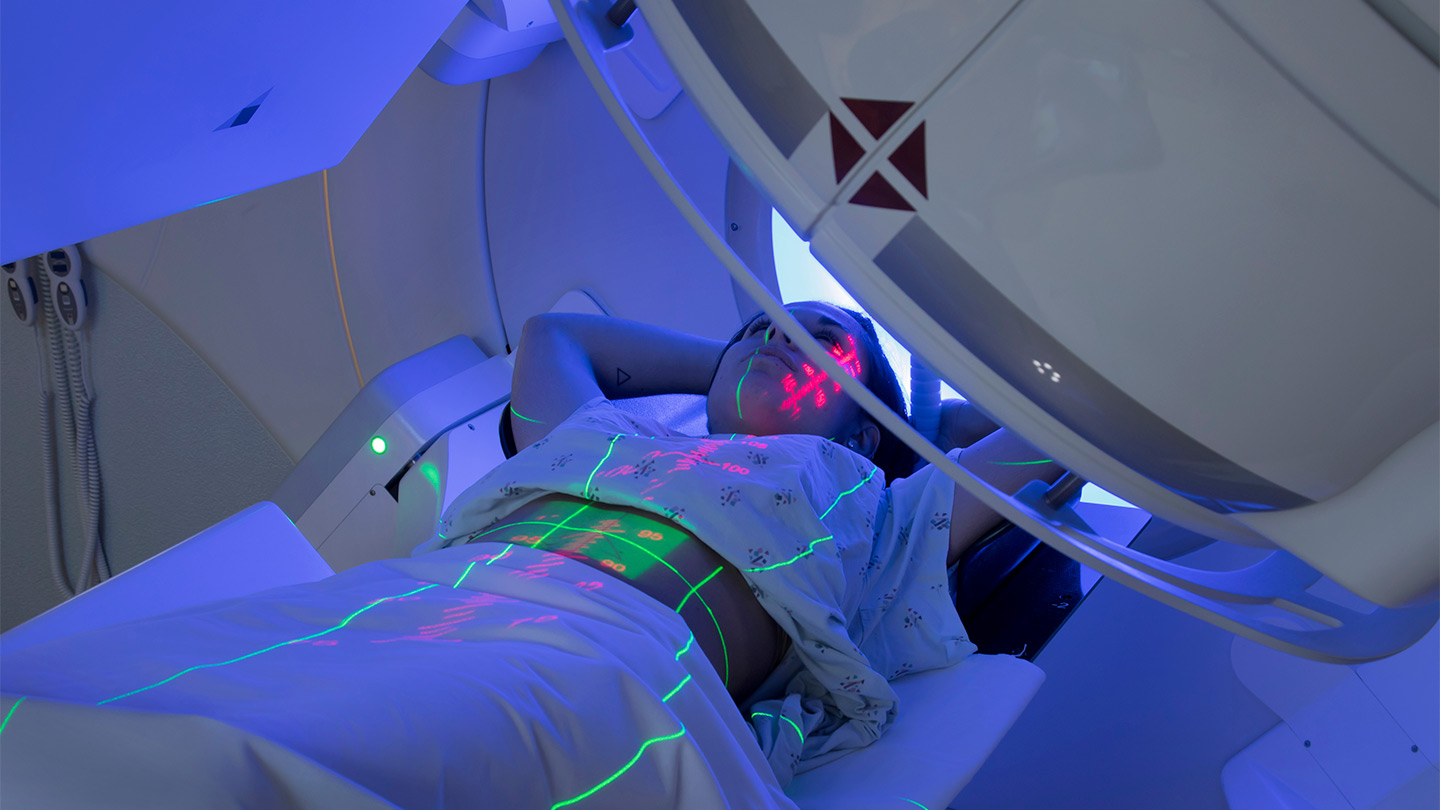
Radiation therapy uses high-energy particles or waves to destroy or damage cancer cells. Radiation is one of the most common treatments for cancer, either by itself or along with other forms of treatment.
In both surgery and radiation, the potential lack of precision makes it difficult to control the boundaries of the treatment which can result in damage to the adjacent nerves, causing urinary incontinence and/or erectile disfunction.
Focal Therapy
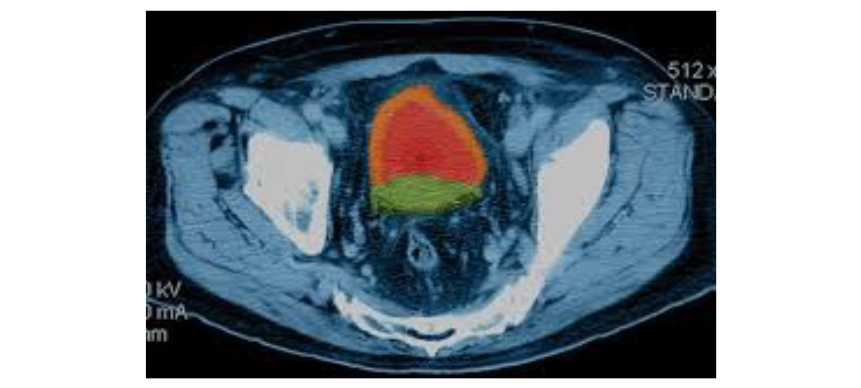
Focal therapy is a minimally invasive method of treatment that can be targeted to destroy only the cancer in the prostate, leaving the remainder of the prostate gland and surrounding important structures like the erectile nerves intact. This treatment reduces the risk of impaired urinary and sexual function compared to other “whole-gland” directed therapies like surgery or radiation therapy. Using MRI fused with real-time ultrasound to target the tumor improves the precision of the technique. Current focal ablation modalities are primarily thermal therapies, either freezing (cryotherapy) or heating (HIFU, single electrode RF, laser, water, steam, electricity).
However, all suffer from the same challenges as the lesion grows from the center outward. The margin of the cancer cell “kill” is indeterminate and difficult to precisely target using thermocouples or imaging. Ablation therefore often extends beyond the necessary area making it difficult to preserve important structures immediately adjacent to tumors such as erectile nerves.
IMAGIN’S CLINICAL SOLUTION
enCAGE Coil™ Precision Ablation System for Prostate Cancer
Focal Therapy with the enCAGE Coil
The enCAGE Coil is being refined to address the limitations of other methods of prostate cancer treatment that can cause impaired urinary and sexual function. The device is a disposable, focal therapy precision ablation system that will delivers bipolar, radiofrequency energy through a distinctive coil electrode, or “cage” and allow the surgeon to preset margins for precise tissue targeting. Similar to the principle of the Faraday Effect, the enCAGE coils retains the RF energy within the confines of the coil, preventing leakage beyond the ablation zone that could otherwise damage adjacent erectile nerves. The procedure is minimally invasive can be performed in a physician’s office.
Two clinical studies were completed on 41 patients by Dr. Samir Taneja, NYU/Langone Medical Center, Dr. Mark Emberton, University College London and Dr. Clement Orczyk, that showed negative biopsy results in 71 and 80% of subjects within six months following treatment, respectively. Results of the University College London study were published in Journal of Urology, May 2018.
The enCAGE received an initial FDA 510(k) clearance in 2008 for percutaneous, laparoscopic, or intraoperative thermal coagulation of soft tissues. Product enhancements are under development. The product has strong intellectual property with 8 issued patents.